autocatalysis and oscillatory reactions
Autocatalysis
A single chemical reaction is said to have undergone autocatalysis, or be autocatalytic, if the reaction product itself is the catalyst for that reaction.
oscillatory reactions
During the course of an oscillatory reaction the concentrations of some species, typically the intermediates, periodically changes. A Belousov–Zhabotinsky reaction, or BZ reaction, is one of a class of reactions that serve as a classical example ofnon-equilibrium thermodynamics, resulting in the establishment of anonlinear chemical oscillator.The Belousov-Zhabotinsky (BZ) reaction is a chemical oscillatory reaction, fluctuating in color. Named after B. P. Belousov (who discovered the reaction) and A. M. Zhabotinsky (who continued Belousov�s early work), the reaction originally consisted of a one-electron redox catalyst, an organic substrate that is easily brominated and oxidized, and a bromate ion in form of NaBrO3 or KBrO3 all dissolved in sulfuric or nitric acid. The typical catalyst that is used is ferroin. Ferroin, in its oxidized state, has a blue color, while in its reduced state ferroin is red. As the BZ reaction alternates between the oxidized state and reduced state, the solution changes its color.
Autocatalytic reactions are those in which at least one of the products is a reactant. Perhaps the simplest autocatalytic reaction can be written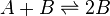
with the rate equations. 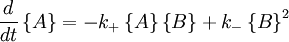
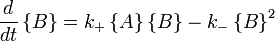 This reaction is one in which a molecule of species A interacts with a molecule of species B. The A molecule is converted into a B molecule. The final product consists of the original B molecule plus the B molecule created in the reaction.
The key feature of these rate equations is that they are nonlinear; the second term on the right varies as the square of the concentration of B. This feature can lead to multiple fixed points of the system, much like a quadratic equation can have multiple roots. Multiple fixed points allow for multiple states of the system. A system existing in multiplemacroscopic states is more orderly (has lower entropy) than a system in a single state.
This reaction is one in which a molecule of species A interacts with a molecule of species B. The A molecule is converted into a B molecule. The final product consists of the original B molecule plus the B molecule created in the reaction.
The key feature of these rate equations is that they are nonlinear; the second term on the right varies as the square of the concentration of B. This feature can lead to multiple fixed points of the system, much like a quadratic equation can have multiple roots. Multiple fixed points allow for multiple states of the system. A system existing in multiplemacroscopic states is more orderly (has lower entropy) than a system in a single state.